Groundwater often has low levels of dissolved oxygen because it is not continually exposed to the atmosphere. When groundwater reaches the surface, the following reaction involving soluble iron(II) can occur. What are the oxidation numbers of the elements in the product, Fe(OH) 3? 4Fe2+(aq) 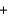 O2(g)
O2(g) 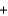 2H2O(l )
2H2O(l ) 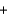 8OH-
8OH- 4Fe(OH) 3(s)
4Fe(OH) 3(s)
Definitions:
Evaluating
The process of assessing or judging the value, significance, or condition of something, typically following certain criteria or standards.
Abnormal Behavior
Actions or behaviors that deviate significantly from what is considered normal or typical.
Personal Distress
A negative emotional response individuals feel when witnessing others in distress, motivating them to alleviate both their own and others' suffering.
Function Normally
The ability of an organism, system, or mechanism to operate or perform within its expected parameters, without impairment or dysfunction.
Q1: Which of the following is the most
Q20: Write the electron configuration of Co<sup>2</sup><font face="symbol"><sup></sup></font>.
Q24: In a demonstration of strong electrolytes, weak
Q84: Which of the following depicts a heterogeneous
Q85: During the expansion of steam inside the
Q101: The pressure gauge on a cylinder used
Q106: Four containers, each with the same volume
Q108: Write the net ionic equation for the
Q115: You have a summer job in a
Q118: Controlling the concentration of ammonia is