Groundwater often has low levels of dissolved oxygen because it is not continually exposed to the atmosphere. When groundwater reaches the surface, the following reaction involving soluble iron(II) can occur. What are the oxidation numbers of the elements in the product, Fe(OH) 3? 4Fe2+(aq) 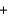 O2(g)
O2(g) 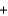 2H2O(l )
2H2O(l ) 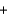 8OH-
8OH- 4Fe(OH) 3(s)
4Fe(OH) 3(s)
Definitions:
Idiosyncrasy Credit
A theoretical concept that describes the tolerance a group has for the unconventional behavior of its members, based on the individual's status and previous contributions to group cohesion or performance.
Charismatic
Possessing a compelling charm or appeal that inspires devotion or admiration from others.
Weber's Definition
Refers to Max Weber's comprehensive descriptions and categorizations in sociology, particularly his definitions of bureaucracy and the process of rationalization.
Adolf Hitler
A German politician and leader of the Nazi Party who rose to power as Chancellor of Germany in 1933 and later Führer in 1934, initiating World War II and enacting policies that led to the Holocaust.
Q16: Write the net ionic equation for the
Q26: When the oxidation reduction reaction shown here
Q56: Which statement regarding combustion of a sample
Q61: Which one of the following is a
Q65: Which letter below represents the halogen group?
Q67: Which one of the following statements is
Q126: A homogeneous mixture of two or more
Q133: In which of the following reaction mixtures,
Q144: What is the formula for calcium nitride?<br>A)CaN<br>B)Ca<sub>2</sub>N<sub>3</sub><br>C)Ca<sub>2</sub>N<br>D)Ca<sub>3</sub>N<sub>2</sub><br>E)CaN<sub>2</sub>
Q157: An unknown gas held in a 25.0