In a self-contained breathing apparatus, potassium superoxide, KO2, reacts with carbon dioxide to form potassium carbonate and oxygen. Identify the limiting reactant and determine the amount of oxygen gas, in moles, that can be produced from 250 g of KO2 and 450 g of CO2. 4KO2(s) 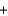 2CO2(g)
2CO2(g)  2K2CO3(s)
2K2CO3(s) 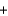 3O2(g)
3O2(g)
Definitions:
Journal Entry
A record in accounting that represents a transaction and its effect on various accounts, ensuring the debits equal the credits.
Present Value
The current value of a future amount of money or stream of cash flows given a specified rate of return.
Compound Interest
This approach to interest calculation includes both the initial principal amount and the compounded interest from earlier stages of a deposit or borrowing.
Bonds Payable
Long-term liabilities representing money a company must pay back to bondholders, typically including principal and interest.
Q6: A range of organic molecules can undergo
Q24: Which of the following is not a
Q25: Hartmann's solution is used in intravenous therapy
Q32: In a mixture of gases, the gas
Q46: According to the first law of thermodynamics,
Q67: Use a graphing utility to graph the
Q81: Determine convergence or divergence of the series.
Q88: You are given a liquid sample that
Q95: What mass of potassium hydroxide is needed
Q138: Locate each element in the periodic table