Metallic sodium was originally produced by the Deville process, where sodium carbonate and carbon black are heated to 1100 C to produce sodium (as a liquid at that temperature) and carbon monoxide gas, according to the following balanced equation.
Na2CO3(s ) 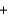 2C(s )
2C(s )  2Na(l)
2Na(l) 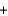 3CO(g)
3CO(g)
If 2.3 kg of sodium carbonate is heated, what is the maximum amount of sodium that can be produced?
Definitions:
Social Conversations
Dialogues between individuals or groups aimed at social interaction rather than transactional or informational exchanges.
Busy
Occupied with tasks or engagements; having a high level of activity, often resulting in limited availability.
Reframing
The practice of changing the way situations or ideas are viewed or interpreted, often to find more positive or functional perspectives.
Conflict Situation
A scenario where opposing interests, beliefs, needs, or values lead to a disagreement or dispute between two or more parties.
Q10: Find the arc length of the given
Q20: Which statement A-D about the reaction of
Q39: The diagram below shows three ion pairs:
Q63: Estimate the error in using <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2342/.jpg"
Q66: Write the balanced reaction equation for the
Q74: Which statement A-D about the reaction of
Q75: What are the units of molar concentration,
Q79: Find a polar equation for the conic
Q100: Given the following figure, which of the
Q140: Ammonia, NH<sub>3</sub>, is an important industrial chemical.