Which statement A-D about the reaction of nitrogen monoxide with oxygen, which is called combustion and is represented below by the following cartoon, is not correct? The reaction product is nitrogen dioxide. 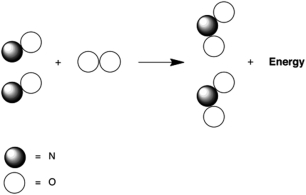
Definitions:
Q1: Give an example of a molecular compound
Q17: Evaluate the integral. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2342/.jpg" alt="Evaluate the
Q26: Show that <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2342/.jpg" alt="Show that
Q26: When the oxidation reduction reaction shown here
Q70: For the given parametric equations, find a
Q82: The number 3.42 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="The number
Q93: Which of the following length measurements
Q95: Write the complete atomic symbol with both
Q103: Identify the oxidizing agent in the following
Q111: Sketch the graph of the polar equation.