One of the buffers that contribute to pH stability in human blood is carbonic acid (H₂CO₃) . Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (HCO₃-) and a hydrogen ion (H⁺) . (See figure.) 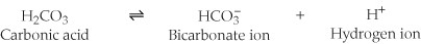 If the pH of blood drops, one would expect ________.
If the pH of blood drops, one would expect ________.
Definitions:
Discontinuous Innovation
Innovations that create major changes in how we live and work by introducing products or services that drastically alter the market.
Continuous Innovation
The ongoing introduction of small and incremental changes or improvements to products, services, or processes over time.
Fridge Apps
Applications designed for smart refrigerators that assist in managing groceries, recipes, and food inventory directly from the fridge's interface.
Discontinuous Innovation
A type of innovation that represents a significant breakthrough or creation of a completely new market, often transforming consumer habits.
Q4: The difference between an aldose sugar and
Q7: A 0.01 M solution of a substance
Q21: Identical heat lamps are arranged to shine
Q27: Visualise the structural formula of each of
Q42: How might a change of one amino
Q42: Which system presents information in a highly
Q50: Which of the following statements is true?<br>A)
Q51: Enzymes that break down DNA catalyse the
Q52: Data about how the present system works
Q64: Some photosynthetic organisms contain chloroplasts that lack