Essay
Instructions: Refer to the data below to answer the following question(s).
of Some Phenols 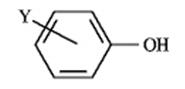
Refer to instructions.
a) The weakest acid in the table is
b) Which of the acids in the table has the weakest conjugate base?
Definitions:
Related Questions
Q5: To answer the questions below, consider the
Q5: Instructions: Refer to the data below
Q10: When graphed,a significant interaction effect produces two
Q11: Instructions: Refer to the data below
Q15: What is the relationship between D-erythrose and
Q20: When a change in the values of
Q20: Which of the following correctly describes a
Q23: Which hydrogen atom(s) in the following compound
Q28: The population mean for the Elementary Tenacity
Q31: Inferential statistics are used to<br>A)decide whether sample