A concentration cell is constructed by using the same half-reaction for both the cathode and anode. What is the value of standard cell potential, E 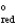 , for a concentration cell that combines a silver anode in contact with 0.10 M silver nitrate and a silver cathode in contact with 0.00003 M silver nitrate? (E
, for a concentration cell that combines a silver anode in contact with 0.10 M silver nitrate and a silver cathode in contact with 0.00003 M silver nitrate? (E 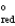 = + 0.80 V for Ag/Ag+)
= + 0.80 V for Ag/Ag+)
Definitions:
Finished Good
A product that has completed the manufacturing process and is ready for sale to consumers or other buyers.
Internal Auditing
The independent and objective evaluation of an organization's financial and operational activities, typically performed by in-house staff.
Construction Firm
A company that specializes in building and designing infrastructure, buildings, and other architectural projects.
Control Problems
Issues arising from the attempt to monitor or regulate processes, people, or systems, which can lead to inefficiency or loss of focus.
Q4: If 500 mL of a solution containing
Q50: Three acids found in foods are lactic
Q52: A disaccharide has a molecular formula of
Q70: Which of the following has set an
Q82: Sulfur dioxide emitted in the burning of
Q82: What are the major factors that affect
Q88: The equilibrium constant for the formation of
Q95: Selenium, an ultratrace element, is used in
Q95: When convertible bonds are first issued:<br>I. the
Q120: The solubility product for an insoluble salt