For the chemical equilibrium aA + bB 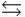 cC, the value of the equilibrium constant, K, is 10. What is the value of the equilibrium constant for the following reaction? cC
cC, the value of the equilibrium constant, K, is 10. What is the value of the equilibrium constant for the following reaction? cC 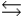 aA + bB
aA + bB
Definitions:
Identity Shift
A process by which an individual's self-perception and presentation to others change, often due to new experiences or roles.
Open Innovation
A business management model that encourages companies to acquire external ideas and internal inventions to accelerate innovation and market adoption.
In-house R&D
Research and Development activities carried out within an organization, as opposed to outsourcing or external collaborations.
Closed Innovation
An innovation approach where all research and development processes are conducted internally within a firm, with little or no input from external sources.
Q4: A saturated compound composed solely of carbon
Q5: Allene has the condensed formula CH<sub>2</sub><br><sub> </sub>
Q13: Which statement about amino acid enantiomers is
Q38: Four solutions have pH values of 3,
Q59: Benzene is a liquid under standard conditions.
Q70: The cyclization of fructose forms a _<br>A)
Q71: An oxygen molecule can have several different
Q84: In a tetrahedral crystal field, _<br>A) d<sub>xy</sub>,
Q85: Given the following data for the reaction
Q102: This is a true story; can you