Gaseous carbon monoxide and hydrogen gas can be used to prepare methanol (CH3OH). Under equilibrium conditions at 427°C, the partial pressures of the components are as follows.
PCO = 1.44 atm 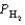 = 4.25 atm
= 4.25 atm 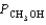 = 2.30 atm
= 2.30 atm
What is the value of the equilibrium constant for the reaction in which one mole of methanol is produced?
Definitions:
Toner Adhesion
The process by which toner powder in laser printers and copiers bonds to the paper, often involving heat and pressure to ensure the print is fixed and durable.
Printer Damage
Physical or functional harm caused to a printing device, often resulting from mishandling, mechanical failure, or external factors.
Personal Checking
A type of bank account that allows individuals to deposit, withdraw, and write checks against their balance for personal, not business, transactions.
Money Market
A section of the financial market where short-term financial instruments with high liquidity are traded.
Q5: Draw a graph of entropy versus temperature
Q28: Which of the following figures illustrates best
Q34: Are the methyl groups in xylene included
Q38: What is the oxidation number of chromium
Q50: Define the term polydentate ligand, and give
Q61: Which of the following molecules is chiral?
Q77: Which of the following monomers is used
Q80: The device in automobiles that has decreased
Q88: Which of the solutions shown here will
Q108: Given that the observed absorption maximum for