The molecular art depicts the following reversible reaction at equilibrium: CO(g) + Cl2(g)  COCl2(g) . What can be inferred about the equilibrium constant, K, for this reaction?
COCl2(g) . What can be inferred about the equilibrium constant, K, for this reaction? 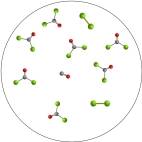
Definitions:
Whiskey Tax
A tax imposed on distilled spirits, notably whiskey, historically significant for inciting the Whiskey Rebellion in the United States in the late 18th century.
Pinckney Treaty
A 1795 agreement between the United States and Spain that defined the borders of the United States with Spanish colonies and granted the U.S. the right to navigate the Mississippi River.
Second Great Awakening
A Protestant religious revival movement in the early 19th century United States, which spurred the growth of social reform movements, such as abolitionism and temperance.
Whiskey Rebellion
A tax protest in the United States in the 1790s, during the presidency of George Washington, triggered by a tax imposed on distilled spirits.
Q1: All p block elements form ions.
Q12: The reactants are favored in the acid-base
Q22: The molecule below is a polar molecule.
Q22: A saline solution used in intravenous drips
Q24: Which of the following is TRUE about
Q43: Which substance is a colloid?<br>A)mayonnaise<br>B)a dental filling<br>C)mint
Q50: How many molecules of ATP are formed
Q54: Which best describes the function of the
Q67: Combustion reactions are redox reactions.
Q72: Three of the four phase changes below