Consider the reversible reaction at equilibrium: N2(g) + O2(g) 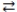 2 NO(g) . What is the effect of removing some N2(g) from the equilibrium system?
2 NO(g) . What is the effect of removing some N2(g) from the equilibrium system?
Definitions:
Neuroglial Cells
Cells in the nervous system that perform supportive and protective roles for neurons, rather than conducting nerve impulses.
Radiotherapy
A treatment method that uses high-energy radiation, commonly for cancer, to damage or destroy abnormal cells or shrink tumors without excessive damage to surrounding healthy tissue.
Sensitive
Refers to the ability to detect or respond to slight changes, signals, or influences.
Moderately
To a certain extent, but not too much; refers to the middle degree or average in actions, quantities, or conditions.
Q18: According to Jacobs,Masson,and Harvill,the most helpful and
Q19: Coenzyme A is a biological oxidizing agent
Q40: What factors should leaders consider when a
Q46: How can leaders use different techniques to
Q47: In which stage of metabolism are biomolecules
Q47: Which salt forms a basic solution when
Q64: The rate of glycolysis decreases when the
Q79: All steps of the citric acid cycle
Q84: A hypotonic solution has _ osmotic pressure
Q84: How many moles of sulfur trioxide