Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3. Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3) 2+: 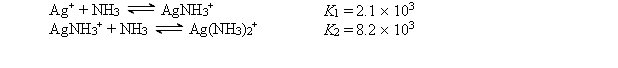 The concentration of Ag+ at equilibrium is
The concentration of Ag+ at equilibrium is
Definitions:
Alternative Dispute Resolution
A method for settling disputes outside of the courtroom, such as through arbitration or mediation.
Mediation
A method of settling disputes outside of court by using the services of a neutral third party, called a mediator. The mediator acts as a communicating agent between the parties and suggests ways in which the parties can resolve their dispute.
International Business Transactions
Economic activities that involve the exchange of goods, services, or information across national borders.
Arbitration Clauses
Provisions in a contract that require disputes to be resolved through arbitration, instead of through court litigation.
Q8: Calculate the concentration of Ag<sup>+</sup> in a
Q12: (Scenario I)This study is best described as:<br>A)
Q15: To establish probabilistic conclusions about the relationship
Q29: 250.0 mL of 1.00 M NaOH<br>A) 10.00<br>B)
Q29: What is the equilibrium expression for the
Q42: You know that people are often more
Q51: A student weighs out 0.568 g of
Q72: After 220.0 mL of 0.200 M NaOH
Q97: 2.42 <font face="symbol"></font> 10<sup>-</sup><sup>3</sup> g of BaSO<sub>4</sub>
Q148: Explain how the solubility of an ionic