The cation M2+ reacts with NH3 to form a series of complex ions as follows: 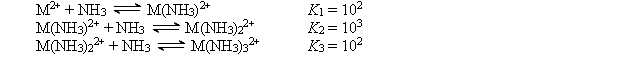 A 1.0 10-3 mol sample of M(NO3) 2 is added to 1.0 L of 15.0 M NH3 (Kb = 1.8 10-5) . Which of the following would be a dominant species in this solution?
A 1.0 10-3 mol sample of M(NO3) 2 is added to 1.0 L of 15.0 M NH3 (Kb = 1.8 10-5) . Which of the following would be a dominant species in this solution?
Definitions:
Lockbox System
A service provided by banks to process payments quickly by allowing companies to have their customers send payments to a special post office box.
Disbursement Float
The time lag between when a payment is issued by a payer and when the funds are actually withdrawn from the payer's account.
Receivable Accounts
Balances of money due to a company for goods or services provided that have not yet been paid by customers.
Collection Time
The average period that a company takes to collect payments from its customers after a sale has been made, impacting cash flow and liquidity.
Q2: Penny is a smoker.Although she has heard
Q11: (Scenario I)Although the results of the study
Q19: Consider the equation 2A(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6420/.jpg" alt="Consider
Q28: The refining of aluminum from bauxite ore
Q41: For the reaction H<sub>2</sub>O(l) <font face="symbol"></font> H<sub>2</sub>O(g)
Q45: A 230.-mL sample of a 0.275 M
Q54: Calculate the pH of a solution made
Q67: Which of the following reactions is associated
Q85: Calculate the temperature at which the average
Q91: First-born children may think they are braver