Consider the following reaction: 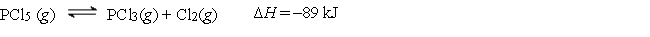
-How can the equilibrium be shifted to the right?
Definitions:
Acid
A substance that releases hydrogen ions when dissolved in water, typically having a pH less than 7 and capable of turning blue litmus paper red.
Alkali
A base that dissolves in water, typically found among the group 1 elements of the periodic table, such as sodium and potassium, which form strongly basic hydroxides.
Ion
A charged atom.
Electron
One of the three major parts of an atom. The electron carries a negative charge.
Q2: A case that is the exception to
Q4: Refer to the following structures. Which of
Q43: Calculate <font face="symbol"></font>H° for the reaction C<sub>4</sub>H<sub>4</sub>(g)
Q48: For the reaction of sodium bromide with
Q65: Which of the following is an incorrect
Q71: Calculate the pH of a 0.50 M
Q89: Nelson is applying for a job within
Q93: If all of the chloride in a
Q99: Sodium perbromate can be synthesized via the
Q147: You have a solution of 0.10 M