Based on the phase diagram shown below, which of the following statements are correct?
I. Sublimation occurs at a point in the transformation that falls along a straight line from point A to point F.
II. C and E represent points where the gas and liquid phases are in equilibrium.
III. (Hvap can be measured at point B) .
IV. Molecules at point D have a greater average kinetic energy than those at point F.
V. The temperature at point E is called the critical temperature of the compound. 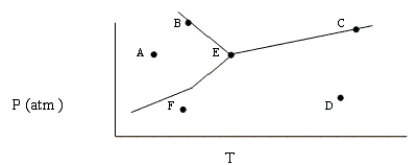
Definitions:
Passing
Presenting yourself as a member of a different group than the stigmatized group to which you belong.
Hegemony
Term developed by Antonio Gramsci to describe the cultural aspects of social control, whereby the ideas of the dominant group are accepted by all.
Discrimination
Unjust or prejudicial treatment of different categories of people, especially on the grounds of race, age, or gender.
Toxic Waste Dumps
Sites designated for the disposal of hazardous materials, which pose risks to environmental health and public safety.
Q13: An isotope of an element is formed
Q21: Which of the following elements has the
Q24: For the reaction<br>2NO(g) + H<sub>2</sub>(g)
Q39: According to crystal field theory, how many
Q42: Choose the element with the smallest ionization
Q46: A metal crystallizes in a body-centered unit
Q57: How many unpaired electrons are found in
Q63: In which case is the bond
Q66: The half-life of <sup>90</sup>Sr is 28.1 years.
Q78: The empirical formula of a compound with