Given below are the temperatures at which two different liquid compounds with the same empirical formula have a vapor pressure of 400 torr. 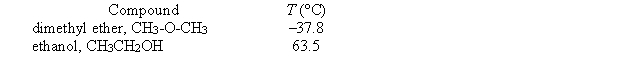 Which of the following statements is false?
Which of the following statements is false?
Definitions:
Levi Strauss
A German-American businessman who founded the first company to manufacture blue jeans, known for his denim pants and contributions to the apparel industry.
Popular Sovereignty
A principle in political theory that the authority of a state and its government is created and sustained by the consent of its people, through their elected representatives.
Prigg v. Pennsylvania
Prigg v. Pennsylvania was a landmark Supreme Court case in 1842 which held that states did not have to enforce the return of runaway slaves, thereby weakening the 1793 Fugitive Slave Act.
U.S. War on Mexico
A military conflict between the United States and Mexico (1846-1848), leading to significant territorial acquisitions by the U.S.
Q13: Of the following, which molecule has the
Q20: Select the correct molecular structure for IF<sub>5</sub>.<br>A)
Q27: For the reaction in which A and
Q28: Which is the symbol for the isotope
Q44: A sample consisting of CO<sub>2</sub>(g) and CO<sub>2</sub>(s)
Q50: Which of the following ions interfere(s) with
Q58: What is the hybridization of the B
Q66: When a 1.50-g sample of glutamic acid
Q85: Which letter shows the activation energy?
Q111: Cesium metal with P<sub>4</sub>(g)