Consider the following Lewis structure. (Lone pairs are not drawn in.) 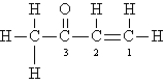 Which statement about the molecule is false?
Which statement about the molecule is false?
Definitions:
Positive Thinking
A mental attitude focusing on the bright side of life and expecting positive results.
Cognitive Theories
Psychological theories emphasizing mental processes such as perception, memory, and problem-solving as sources of understanding behavior and mental state.
Negative Thinking
A cognitive process characterized by pessimistic attitudes and expectations about oneself or the world.
Hopeless Style
An individual's tendency to perceive negative events as unavoidable and unchangeable, leading to feelings of despair.
Q3: In which reaction is <font face="symbol"></font>S° expected
Q21: The standard free energy of formation of
Q21: What form will the pseudo-rate law have?<br>A)
Q33: Which of the following result(s) in an
Q53: Which of the following is paramagnetic?<br>A) N<sub>2</sub><br>B)
Q65: Choose the species with the highest boiling
Q77: Copper is electroplated from an aqueous CuSO<sub>4</sub>
Q79: CH<sub>4</sub><br>A)Give the shape of the molecule.<br>B)Indicate the
Q82: The following questions refer to the hypothetical
Q93: A 0.2 molar solution of a solute