A strip of copper is placed in a 1 M solution of copper nitrate, and a strip of silver is placed in a 1 M solution of silver nitrate. The two metal strips are connected to a voltmeter by wires, and a salt bridge connects the solutions. The following standard reduction potentials apply: 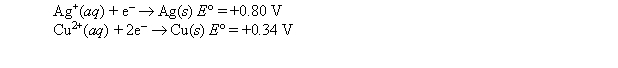 When the voltmeter is removed and the two electrodes are connected by a wire, which of the following does not take place?
When the voltmeter is removed and the two electrodes are connected by a wire, which of the following does not take place?
Definitions:
Real Rate
The interest rate that has been adjusted to remove the effects of inflation, showing the true cost of borrowing.
Economic Rent
A payment made for the use of a good, service, or resource above and beyond what is necessary to encourage its production or supply.
Nonreproducible Resources
Natural resources that cannot be replaced or regrown at a pace that matches their consumption.
Perfectly Elastic
Refers to a situation in demand or supply where quantity changes infinitely with any change in price.
Q3: When an offeree dies before accepting an
Q24: The C<font face="symbol"></font>C<font face="symbol"></font>H bond angles in
Q29: If four orbitals on one atom overlap
Q34: Andrew, a newly graduated lawyer, failed to
Q38: The Queen's Wardrobe, a dressmaking boutique, agrees
Q43: Jolene, aged 16, is hired by the
Q44: A sample consisting of CO<sub>2</sub>(g) and CO<sub>2</sub>(s)
Q58: w is<br>A) less than zero.<br>B) greater than
Q60: Which of the following molecules has a
Q87: How many f orbitals have the value