Based on the phase diagram shown below, which of the following statements are correct?
I. Sublimation occurs at a point in the transformation that falls along a straight line from point A to point F.
II. C and E represent points where the gas and liquid phases are in equilibrium.
III. ΔHvap can be measured at point B.
IV. Molecules at point D have a greater average kinetic energy than those at point F.
V. The temperature at point E is called the critical temperature of the compound. 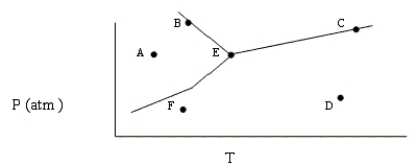
Definitions:
Q13: What is the overall order of the
Q15: How many electrons are in the Lewis
Q28: Pure N<sub>2</sub>O<sub>3</sub> was placed in a vessel
Q36: A sample consisting of CO<sub>2</sub>(g) and CO<sub>2</sub>(s)
Q38: The ratio of the atomic radius to
Q40: Select the correct molecular structure for XeF<sub>4</sub>.<br>A)
Q50: The following questions refer to the hypothetical
Q52: For the reaction A + B →
Q81: The C-C-H bond angles in ethylene, C<sub>2</sub>H<sub>4</sub>,
Q118: _ phosphorus has a regular crystalline structure,