Consider the following Lewis structure. (Lone pairs are not drawn in.) 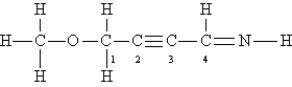 What are the hybridizations of the oxygen atom and of carbon atoms 1, 2, and 4, respectively (order: O C-1 C-2 C-4) ?
What are the hybridizations of the oxygen atom and of carbon atoms 1, 2, and 4, respectively (order: O C-1 C-2 C-4) ?
Definitions:
Long-term Debt
Loans or other forms of credit that are due for repayment in more than one year's time.
Long-term Debt
Borrowings of a company that are due for repayment more than one year into the future.
Current Liabilities
Financial obligations that a company is expected to settle within a year, including accounts payable, short-term loans, and accrued expenses.
Sales Revenue
Earnings a company secures through the sale of goods or services provided.
Q5: Lead metal with H<sup>+</sup>(aq)
Q44: What is the electron configuration for Cr<sup>2+</sup>?<br>A)
Q51: A certain solid substance that is very
Q55: What is the valence electron configuration of
Q56: For the reaction<br>CH<sub>3</sub>CHCH<sub>2</sub>(g) + HCl(g) → CH<sub>3</sub>CHClCH<sub>3</sub>(g)<br>a
Q69: For the process X<sup>-</sup>(g) → X<sup>-</sup>(aq), select
Q76: What is the oxidation state of Mn
Q77: ΔS<br>A) 0<br>B) 103 J/K•mol<br>C) -79.6 J/K•mol<br>D) 79.6
Q81: The standard free energy of formation of
Q109: When molten sulfur reacts with chlorine gas,