Consider the galvanic cell shown below (the contents of each half-cell are written beneath each compartment) . 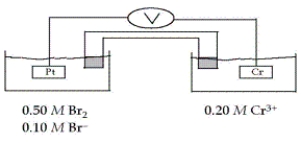 The standard reduction potentials are as follows:
The standard reduction potentials are as follows: 
-Which of the following statements about this cell is false?
Definitions:
Passive Voice
A sentence construction where the subject is acted upon by the verb, often obscuring who is performing the action.
Assigning Blame
The act of attributing responsibility for a fault or wrong to a person or entity.
Present Tense
The verb tense used to describe actions currently happening or general truths.
Traditional Term Papers
Extended essays or research papers written by students over an academic term, reflecting their understanding of a subject.
Q3: The solubility of M(OH)<sub>2</sub> in 0.010 M
Q24: Gold is produced electrochemically from an aqueous
Q33: How many electrons in an atom can
Q43: Calculate the entropy change when 5.00 mol
Q44: The concentration of Al<sup>3+</sup> in a saturated
Q44: Which of the following is a conjugate
Q46: An element E has the electron configuration
Q75: Select the correct molecular structure for IF<sub>5</sub>.<br>A)
Q86: In which of the following cases must
Q170: The solubility, in moles per liter, of