Consider the galvanic cell shown below (the contents of each half-cell are written beneath each compartment) . 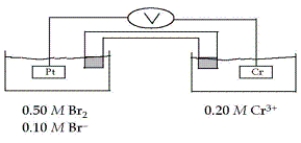 The standard reduction potentials are as follows:
The standard reduction potentials are as follows: 
What is the value of E for this cell at 25°C?
Definitions:
Revenue Per Employee
A financial metric indicating how much revenue each employee generates for the business, often used to assess efficiency and productivity.
LLC Members
Individuals or entities that hold ownership in a Limited Liability Company, sharing in the profits and losses according to the terms outlined in the LLC operating agreement.
Business Expansion
The process of increasing the size, output, or scope of a company's operations, typically requiring additional resources and strategies.
Partnership Liquidation
The process of closing a partnership by selling off assets, paying liabilities, and distributing the remaining assets to partners.
Q1: Which of the following statements about this
Q5: Consider the following reduction potentials: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg"
Q15: 1s<sup>2</sup>2s<sup>2</sup>2p<sup>5</sup>
Q15: Which of the following is diamagnetic?<br>A) F<sub>2</sub><sup>+</sup><br>B)
Q16: What is the electron configuration of indium?<br>A)
Q18: C<sub>2</sub>H<sub>5</sub>OH(l) + 3O<sub>2</sub>(g) → 2CO<sub>2</sub>(g) + 3H<sub>2</sub>O(l),
Q43: What is the value of the equilibrium
Q58: The number of unpaired electrons in the
Q61: Consider the molecular-orbital description of the NO<sup>-</sup>
Q79: A monoprotic weak acid, when dissolved in