A certain chemical reaction sets up an equilibrium as follows
3A2(aq) + 2B3(aq) 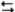 6AB(aq)
6AB(aq)
After being started with unknown amounts of A2 and B3. Once the system reaches equilibrium the concentrations of A2, B3, and AB are precisely determined and the temperature recorded. What other quantities can be determined? Q 0 is the reaction quotient at the initial conditions.
Definitions:
Percentiles
A measure that indicates the value below which a given percentage of observations in a group of observations falls.
Stanines
Derived from the term “standard nines,” this is a standardized score frequently used in schools. Often used with achievement tests, stanines have a mean of 5 and a standard deviation of 2, and range from 1 to 9.
T-scores
Standardized scores with a mean of 50 and a standard deviation of 10, used to compare an individual's score against a normative sample.
Grade Equivalents
A metric used in education to compare a student's test score with the average scores of students in other grades.
Q22: Consider an electrochemical cell with a copper
Q26: For a certain process, at 319 K,
Q32: For the elements Rb, F, and O,
Q47: Consider the reaction<br>CaCl<sub>2</sub>(s) + 2H<sub>2</sub>O(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg"
Q72: A cell is set up with copper
Q83: Which of the following exhibits the correct
Q86: Calculate the pH of a solution that
Q92: For an electron in a 2.00-nm one-dimensional
Q119: The cation M<sup>2+</sup> reacts with NH<sub>3</sub> to
Q173: Consider a solution of 2.0 M HCN