A 50.00-mL sample of 0.100 M Ca(NO3) 2 is mixed with 50.00 mL of 0.200 M NaF. When the system has come to equilibrium, which of the following sets of conditions will hold? The Ksp for CaF2 is 4.0 × 10-11. 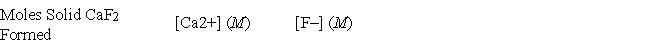
Definitions:
Q11: Consider the reaction<br>C<sub>2</sub>H<sub>5</sub>OH(l) + 3O<sub>2</sub>(g) → 2CO<sub>2</sub>(g)
Q13: Which atomic species, in its ground state,
Q35: The acids HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub> and HF are both
Q37: In a common car battery, six identical
Q45: Calculate ΔS for cooling 2.60 mol of
Q60: In the reaction<br>2MnO<sub>4</sub><sup>-</sup> + 5H<sub>2</sub>O<sub>2</sub> + 6H<sup>+</sup>
Q64: Given the following information, determine the standard
Q65: If you know K<sub>b</sub> for ammonia, NH<sub>3</sub>,
Q68: Identify the strongest acid.<br>A) HNO<sub>3</sub><br>B) HCN<br>C) OH<sup>-</sup><br>D)
Q79: Consider a sample of neon gas in