You have a solution of 0.10 M Cl- and 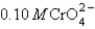 . You add 0.10 M silver nitrate dropwise into the solution. Ksp for Ag2CrO4 is 9.0 × 10-12 and for AgCl is1.6 × 10-10. Which of the following will precipitate first?
. You add 0.10 M silver nitrate dropwise into the solution. Ksp for Ag2CrO4 is 9.0 × 10-12 and for AgCl is1.6 × 10-10. Which of the following will precipitate first?
Definitions:
Error
The difference between the observed value and the true value due to measurement or estimation inaccuracies.
Multiple Regression Analysis
A method in statistics that employs multiple predictor variables to estimate the result of a dependent variable.
SSE
Sum of Squared Errors, a measure of the discrepancy between the data and an estimation model, indicating the total deviation of data points from the fitted values.
Estimated Income
An approximation of the amount of money expected to be earned over a specific period.
Q1: A 25.0 g piece of aluminum (which
Q7: What is the oxidation state of chromium
Q20: Calculate the pH of a solution made
Q28: Which of the following statements is/are true
Q45: The equilibrium constant for A + 2B
Q49: A 50.0-mL sample of 2.0 × 10<sup>-4</sup>
Q55: Which of the following statements is/are true
Q56: Volume versus temperature in degrees Celsius for
Q79: A bomb calorimeter has a heat capacity
Q99: You have a solution of 0.10 M