Short Answer
A certain element (Z) reacts to form three gaseous compounds. Consider the following data (pressure = 1.0 atm). 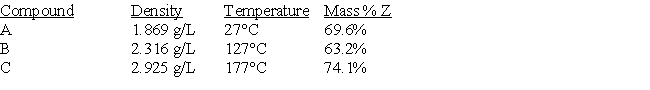
Determine the identity of element Z.
Definitions:
Related Questions
Q6: hydrosulfuric acid
Q9: For which gas do the molecules have
Q16: What is [OH<sup>-</sup>] in a 0.50 M
Q31: Consider the following reaction:<br>4NH<sub>3</sub>(g) + 7O<sub>2</sub>(g) →
Q32: sodium dichromate
Q34: A vessel with a volume of 10.1
Q47: Consider the following standard heats of formation:<br>P<sub>4</sub>O<sub>10</sub>(s)
Q115: Which titration curve would result from the
Q120: The pH of a 0.100 M solution
Q121: Calculate the pH of a 1.8 M