How many electrons are transferred in the balanced redox equation that results from combining these two half-reactions on a molar scale?
Al 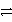
Al3+ + 3e- and Cu2+ + 2 e- 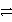
Cu
Definitions:
Receptive Fields
Specific areas within the sensory nervous system that, when stimulated, affect the activity of neurons.
Preexisting Categories
Cognitive classifications or groups that an individual has already formed before encountering new information, which helps in organizing and assimilating new data.
ΔI
Signifies a change in a variable, commonly used in mathematics and physics to denote a change in intensity or other measurable factors.
Standard Stimulus
A specific, unchanging stimulus used as a basis for comparison in experimental investigations.
Q1: What is the ground state electron configuration
Q4: A contract for sale or for testamentary
Q7: Which of the following is the best
Q13: Which of the following is the primary
Q14: Under the Bankruptcy Act, secured creditors get
Q14: Which off the following provides the conversion
Q25: Which of the following is the best
Q28: Consider the following model. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6511/.jpg" alt="Consider
Q34: Which of the following combinations of reactant
Q42: Two natural isotopes exist for silver.51.83% of