Consider the following table of relative strengths of oxidizing and reducing agents:
Oxidizing Agent
Reducing Agent
Stronger
A+ + e- 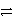
A
Weaker
B2+ + 2 e- 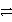
B
Weaker
C3+ + 3 e- 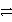
C
Stronger
Which of the following is the correct net ionic equation for the redox reaction between A+ and C and the correct direction that is favored?
Definitions:
Eyewitness Memory
The recollection by individuals of events they have witnessed, especially significant to criminal investigations.
Laboratory Experiments
Controlled tests or investigations conducted in a specially equipped space where variables can be manipulated and measured to explore scientific theories.
Real-World Stimuli
External inputs from the environment that can be perceived and interacted with in everyday life.
Eyewitness Memory
The recall of individuals who have witnessed a particular event or crime, concerning their ability to remember and report details accurately.
Q1: Tenant's fixtures cannot be transferred when the
Q6: Consider a general comparison between acid-base and
Q14: Consider the following general wedge-and-dash diagrams. (i)<img
Q18: Consider the open-end manometer shown below. <img
Q19: Calculate the molar mass of molecular iodine.<br>A)126.9
Q25: What is the empirical formula of a
Q28: How many of the following elements belong
Q31: A _ gift is made by someone
Q35: Which is the correct net ionic equation
Q38: In deciding whether a bond is polar