How many electrons are transferred in the balanced redox equation that results from combining these two half-reactions on a molar scale?
Al 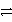
Al3+ + 3e- and Cu2+ + 2 e- 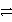
Cu
Definitions:
Performance Measure
Metrics used to evaluate the effectiveness, efficiency, and progress of a project or employee towards achieving objectives.
Cost Per Action
A digital advertising payment model where advertisers pay for a specific action, such as a sale, click, or form submission, tied directly to their advertisement.
Cost Per Click
A digital advertising metric that calculates the amount paid by an advertiser for each click on their ads.
Cost Per Thousand
A metric used to price advertising, measuring the cost to reach a thousand viewers or impressions in a given medium.
Q2: Which of the following is true of
Q2: A book is pushed off a table,and
Q9: What is the volume of 22 g
Q16: The order of increasing principal energy levels
Q17: Choose the correct equilibrium constant expression for
Q17: How many moles of oxygen are consumed
Q31: Calcium reacts with hydrobromic acid.Write the balanced
Q33: What happens in a galvanic cell?<br>A)Cations are
Q35: Consider the diagram given below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6511/.jpg"
Q35: Which of the following features of Dalton's