H = +572 kJ for the decomposition of water by the reaction 2 H2O( 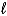 ) 2 H2(g) + O2(g) .How many grams of water can be decomposed by 5.00 *103 kJ of energy?
) 2 H2(g) + O2(g) .How many grams of water can be decomposed by 5.00 *103 kJ of energy?
Definitions:
Ending Work in Process
The value of goods that are in the process of being manufactured but are not yet complete at the end of an accounting period.
Materials
Refers to the raw inputs, supplies, or components used in the manufacturing or production process to create finished goods.
Unit Conversion Cost
The cost incurred to transform raw materials into finished goods on a per unit basis, including both direct labor costs and manufacturing overhead.
Materials Cost
The total expense incurred for materials that are used in the production of goods or services.
Q1: Which of the following substances is/are heterogeneous?<br>(i)Gasoline<br>(ii)A
Q2: Which of the following is the best
Q4: A contract for sale or for testamentary
Q6: Consider the particulate level representation of a
Q8: The process by which a security interest
Q9: The removal of a plant from one
Q18: California law does not allow for a
Q24: What is the significance of Section 8b)4)
Q25: Non-registration of the partnership name will violate
Q35: Which of the following molecules is/are polar?<br>(i)