Which of the following statements is always true concerning a reaction represented by the following balanced chemical equation? 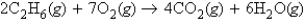
Definitions:
Critical Stages
Key periods in development or processes where important changes or decisions result in significant impact on outcomes.
Conceptual Webs
Networks of related concepts that facilitate understanding and learning by organizing knowledge in interconnected ways.
Categorical Dimensions
A way of representing variables or data that are divided into categories or groups based on specific characteristics.
Social Learning Theory
A theory positing that people learn from one another, via observation, imitation, and modeling, emphasizing the importance of societal and environmental factors in influencing behavior.
Q7: The correct formula for titanium(IV) oxide is<br>A)
Q7: Name the type of crystalline solid formed
Q22: All these atoms have seven electrons in
Q24: Consider the following compounds: CO NH<sub>3</sub> CO<sub>2</sub>
Q25: Calculate the number of moles of CH<sub>4</sub>
Q32: Perform the following conversion of pressure units:
Q44: You have 12.6 g of an unknown
Q47: The greenhouse effect occurs only on the
Q75: Which of the following has a double
Q75: Consider that calcium metal reacts with oxygen