Which of the following statements is always true concerning a reaction represented by the following balanced chemical equation? 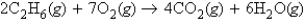
Definitions:
Efficiency
The ability to achieve a goal or perform a task with the minimum expenditure of time and resources.
Persuasive Technique
Strategies used to convince or influence others to adopt a certain belief or take action, often involving emotional or logical appeals.
Writer Benefits
Advantages or positive outcomes that authors or writers receive from their craft, such as recognition, income, or personal satisfaction.
Primary Channel
The main method or medium through which communication, information, or products are delivered to a target audience.
Q13: When the following equation is balanced
Q15: Balance the equation<br>CaCO<sub>3</sub>(s) + HCl(aq)
Q28: Balance the equation<br>C<sub>6</sub>H<sub>14</sub> + O<sub>2</sub>
Q35: You carry out the reaction represented
Q51: Draw the Lewis structure for CCl<sub>4</sub>.
Q53: Calculate the empirical formula of a compound
Q55: C<sub>3</sub>H<sub>8</sub>(g) + 5O<sub>2</sub>(g) <span class="ql-formula"
Q63: Balance the equation<br>C<sub>8</sub>H<sub>18</sub>(l) + O<sub>2</sub>(g)
Q75: What is the formula for potassium carbonate?<br>A)
Q80: There are two balloons, both the same