During transfer of two electrons through the mitochondrial respiratory chain, the overall reaction is:
NADH + 1/2 O2 + H+ NAD+ + H2O
For this reaction, the difference in reduction potentials for the two half-reactions ( E'°) is +1.14 V. Show how you would calculate the standard free-energy change, G'°, for the reaction. (The Faraday constant, 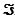 , is 96.48 kJ/V · mol.)
, is 96.48 kJ/V · mol.)
Definitions:
In The Money
A term used in options trading to describe a situation where an option has intrinsic value, indicating a favorable position.
Employee Stock Option (ESO)
A benefit given to employees which grants them the right to purchase shares of the company at a specified price after a certain period.
Call Options
Financial contracts giving the option buyer the right, but not the obligation, to buy a stock, bond, commodity, or other assets at a specified price within a specific time period.
Q5: Which of the following is true about
Q18: The enzyme glycogen phosphorylase:<br>A) catalyzes a
Q26: Which part of the citric acid cycle
Q32: Provide a brief explanation for the observation
Q56: The reaction of the citric acid
Q75: After a meal rich in carbohydrates, which
Q82: _ is a product of pyruvate in
Q93: Arsenic inhibits the pyruvate dehydrogenase complex
Q100: Epinephrine binding to <span class="ql-formula"
Q101: Which statement about facilitated diffusion across a