Considering the limiting reactant,what is the mass of zinc sulfide (97.46 g/mol) produced from 0.750 g of zinc and 0.750 g of sulfur? Zn(s) + S(s) 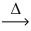 ZnS(s)
ZnS(s)
Definitions:
Increasing Mobility
refers to the process of enhancing one's ability to move freely and easily, often in the context of physical therapy or rehabilitation.
Nursing Outcomes Classification
A systematic organization of nurse sensitive outcomes into groups or categories based upon similarities, dissimilarities, and relationships among the outcomes.
Likert Scale
A measurement scale with multiple answer options that measures respondents' attitudes or opinions.
Priming
Priming is a psychological term referring to the process by which exposure to a stimulus influences a response to a subsequent stimulus, without conscious guidance or intention.
Q52: The phosphite ion,PO₃3-,is classified as which of
Q55: How many moles of water are produced
Q59: If we have enough participants in our
Q70: What is the number of magnesium atoms
Q85: Given a mole of steel shotput balls,which
Q102: What is the molar mass of ammonium
Q156: Subjects are randomly assigned to the experimental
Q161: Octane,C8H18,is a major component in gasoline.What is
Q169: When two variables are not related,the correlation
Q200: A research hypothesis proposes that consuming low-carbohydrate