Considering the limiting reactant,what is the volume of NO gas produced from 30.0 L of nitrogen gas and 20.0 L of oxygen gas? (Assume constant conditions. ) N₂(g) + O₂(g) 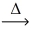 2 NO(g)
2 NO(g)
Definitions:
Technical Eclecticism
An approach in therapy that utilizes techniques from various psychological theories based on their effectiveness for specific problems, rather than adhering to a single theoretical framework.
Theoretical Principles
Fundamental concepts or assumptions that form the basis for theories, guiding research and practice in various academic and professional fields.
Techniques
Specific methods or strategies used in the execution of a task, often referring to the practical application of theories in various fields.
Post-modernism
Post-modernism in the context of psychology and therapy challenges absolute truths, emphasizing the subjective nature of human experience and the importance of multiple perspectives.
Q36: The term _ is unregulated and so
Q44: Butyric acid is the odor of rancid
Q51: What is the Stock system name for
Q51: Which of the following represents 1 mol
Q68: Research methods that describe behavior,but are not
Q86: Assuming similar conditions,how many liters of carbon
Q97: How many moles of argon gas contain
Q114: Considering the limiting reactant,what is the volume
Q155: A researcher plans to conduct an experiment
Q187: Dr.Littman-Smith is conducting research in Kenya in