Considering the limiting reactant,what is the volume of NO₂ gas produced from 3.00 L of NO gas and 2.00 L of oxygen gas? (Assume constant conditions. ) 2 NO(g) + O₂(g) 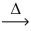 2 NO₂(g)
2 NO₂(g)
Definitions:
References
A list of sources or citations used to support information, assertions, or ideas presented.
Senior Accountant
An experienced accounting professional responsible for managing complex financial records, audits, and reports within an organization.
U.S.
A country located in North America, officially known as the United States of America, characterized by a federal republic form of government.
Specific Instructions
Detailed directions or orders given to guide someone in performing a particular task or procedure.
Q2: Which of the following produces the characteristic
Q10: How many moles of hydrogen peroxide decompose
Q18: What are the products from the following
Q43: A nitrogen atom has a radius of
Q66: Considering the limiting reactant,what is the mass
Q87: Sigmund Freud argued that many of his
Q96: What is the mass of 455 mL
Q98: Which of the following is not a
Q105: Milgram concluded that personality traits were virtually
Q151: What is the chemical formula for strontium