Considering the limiting reactant,what is the volume of NO gas produced from 50.0 L of ammonia gas and 60.0 L of oxygen gas? (Assume constant conditions. ) 4 NH₃(g) + 5 O₂(g) 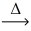 4 NO(g) + 6 H₂O(g)
4 NO(g) + 6 H₂O(g)
Definitions:
Tetanus Toxoid
A vaccine used to provide immunity against tetanus by inducing the body to produce antibodies against the tetanus toxin.
Ice Pack
A pack filled with ice or a gel substance that's used to reduce swelling, pain, or to cool a body part.
Choking
An emergency situation where the airway is blocked, preventing normal breathing.
Severed Ear
A medical condition or injury involving complete or partial detachment of the ear from the rest of the head.
Q7: Which of the following solid compounds is
Q28: One gram of silver contains 5,580,000,000,000,000,000,000 atoms.Express
Q42: What is the systematic name for NH₄+?<br>A)ammonium
Q55: What is the coefficient of sodium metal
Q80: Considering the limiting reactant,what mass of cobalt(III)sulfide
Q85: Given a mole of steel shotput balls,which
Q103: If a rocket shell packed with fireworks
Q116: Which of the following solid compounds is
Q150: Which of the following solid compounds is
Q158: Margie is trying to define "anxiety" in