Multiple Choice
What volume of oxygen gas reacts with 5.00 mL of methane,CH₄? (Assume temperature and pressure remain constant. )
__CH₄(g) + __O₂(g) 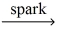 __CO₂(g) + __H₂O(g)
__CO₂(g) + __H₂O(g)
Definitions:
Related Questions
Q2: When Oliver finds out that the average
Q9: What is the term for a systematic
Q9: What is the volume of carbon dioxide
Q20: Aqueous H₂Se is classified as which of
Q25: What is the volume occupied by 0.155
Q26: What is the volume of oxygen gas
Q35: Which of the following is used to
Q56: What is the Stock system name for
Q112: How many moles of oxygen gas react
Q117: What is the mass in grams of