Considering the limiting reactant concept,how many moles of cobalt(III) oxide are produced from the reaction of 1.00 mol of cobalt and 1.00 mol of oxygen gas? 4 Co(s) + 3 O₂(g) 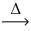 2 CO₂O₃(s)
2 CO₂O₃(s)
Definitions:
Automatically Logs
The process by which a system records events, transactions, or activities without manual intervention.
University Computer
A computer provided or used within a university setting, often for academic or administrative purposes.
Biometrics
The science and technology of authentication (i.e., establishing the identity of an individual) by measuring the subject’s physiological or behavioral characteristics.
User
An individual or entity that utilizes software, a system, or a service for their own purposes or benefit.
Q3: If the density of an unknown gas
Q24: A sodium atom has a radius of
Q80: The first psychological laboratory was officially established
Q83: Round off the following measurement to three
Q87: What is the chemical formula for mercuric
Q103: Dennis believes that women are worse drivers
Q107: Considering the limiting reactant concept,how many moles
Q113: Research suggests that you are more likely
Q152: Benevolent sexism is affectionate,but patronizing.
Q161: Evolutionary psychology is a part of which