Considering the limiting reactant,what is the mass of iron produced from 75.0 g of iron(II) oxide (71.85 g/mol) and 25.0 g of magnesium metal? FeO(l) + Mg(l) 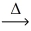 Fe(l) + MgO(s)
Fe(l) + MgO(s)
Definitions:
Principles Application
The act of applying fundamental truths or propositions that serve as the foundation for a system of belief or behavior.
Partnerships
An official business operation conducted by two or more people, with a shared management system and profit allocation.
Double Taxation
The imposition of two or more taxes on the same income, asset, or financial transaction.
Limited Liability Company
A commercial foundation that unifies a corporation's limited liability with the direct tax benefits characteristic of partnerships or sole proprietorships.
Q10: Entrapment occurs when someone in authority asks
Q25: How many significant digits are in the
Q34: What is the systematic name for Br3O8?<br>A)bromine
Q58: What does it mean to say that
Q70: What is the term for a system
Q108: Dawn is systematically recording behaviors at a
Q126: What type of chemical reaction is illustrated
Q137: Which of the following is evidence for
Q150: Which of the following solid compounds is
Q172: A business owner records the amount of