Considering the limiting reactant,what is the mass of iron produced from 80.0 g of iron(II) oxide (71.85 g/mol) and 20.0 g of magnesium metal? FeO(l) + Mg(l) 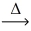 Fe(l) + MgO(s)
Fe(l) + MgO(s)
Definitions:
Drug Intoxication
The physical and mental state caused by the consumption of substances at levels that impair functioning.
Limbic System
A complex system of nerves and networks in the brain, involved in the control of basic emotions and drives.
Unfulfilled Wishes
Unfulfilled wishes are desires or aspirations that have not been realized or achieved.
Manifest Content
In psychoanalytic theory, the literal surface meaning or storyline of a dream as remembered by the dreamer.
Q11: What is the general term for any
Q47: Randy meets a Californian who grows all
Q57: What is the formula of the predicted
Q79: How many significant digits are in the
Q80: The first psychological laboratory was officially established
Q86: Round off the following measurement to three
Q87: How many atoms of nickel equal a
Q88: What is the coefficient of hydrogen gas
Q107: Considering the limiting reactant concept,how many moles
Q109: The ability of a test to measure