Considering the limiting reactant,what is the volume of NO gas produced from 60.0 L of ammonia gas and 50.0 L of oxygen gas? (Assume constant conditions. ) 4 NH₃(g) + 5 O₂(g) 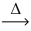 4 NO(g) + 6 H₂O(g)
4 NO(g) + 6 H₂O(g)
Definitions:
Null Hypothesis
The hypothesis that there is no significant difference or effect, serving as the default assumption to be tested against the alternative hypothesis.
Population Proportions
The ratio of members in a statistical population that have a particular attribute or characteristic.
Type I Error
A statistical error that occurs when a true null hypothesis is incorrectly rejected.
Type II Error
The error that occurs when a statistical test fails to reject a false null hypothesis, incorrectly concluding that there is no effect or difference when there is.
Q15: Which of the following is true about
Q21: Considering the limiting reactant concept,how many moles
Q26: What is the volume of oxygen gas
Q29: An operational definition is<br>A)a statement that attempts
Q36: What is the mass of hydrogen gas
Q72: Ralph seeks a psychiatrist and begins to
Q75: Subtract 10.0 mL from 45.65 mL and
Q79: Predict the chemical formula for gallium sulfide,given
Q130: Which of the following metals reacts with
Q200: A research hypothesis proposes that consuming low-carbohydrate