Which of the statements listed below is true regarding the following redox reaction occurring in a nonspontaneous electrochemical cell? 3 C(s) + 2 CO₂O₃(l) 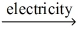 4 Co(l) + 3 CO₂(g)
4 Co(l) + 3 CO₂(g)
Definitions:
Producer Surplus
The difference between what producers are willing to sell a product for and the price they actually receive.
Equilibrium Price
The selling price where the quantity of goods on offer is equal to the quantity consumers want to buy.
Consumer Surplus
The variance between the sum consumers are willing to shell out for a good or service and the sum they actually shell out.
Equilibrium Price
The price at which the quantity of a good or service demanded equals the quantity supplied, leading to a balance in the market.
Q1: What is the color of bromthymol blue
Q19: Which of the statements listed below is
Q23: What substance is oxidized in the following
Q44: Apply the like dissolves like rule to
Q60: What is the general equilibrium constant expression,Keq,for
Q62: Which of the following is equivalent to
Q79: What is the mass of chlorine in
Q81: When potassium iodide,KI,dissolves in water,which of the
Q124: What is the term for a nuclear
Q128: Which of the following techniques can be