Write an expression for the equilibrium constant (Keq) for the following reaction.
NH₃ (g) + H₂O (l) 
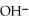 (aq) +
(aq) + 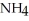 + (aq)
+ (aq)
Definitions:
Regulatory Efficiency
The effectiveness and efficiency with which regulations are designed and implemented, minimizing costs and burdens to businesses while accomplishing regulatory goals.
Investment Freedom
Investment freedom refers to the ease with which individuals and corporations can move their resources into and out of a country's financial market without encountering barriers or restrictions.
Financial Freedom
The state of having sufficient personal wealth to live, without having to work actively for basic necessities, allowing an individual to enjoy life and pursue personal interests.
Risk Insurance
A financial product or service that provides protection against potential future losses or damages, typically involving the transfer of risk from an individual or business to an insurance company in exchange for premiums.
Q2: uranium-238<br>A)thorium-234<br>B)flerovium-286<br>C)radon-220<br>D)lead-206<br>E)bohrium-262<br>F)meitnerium-275
Q5: A sodium hydroxide solution is a non-electrolyte.
Q7: DNA molecules contain codes from which proteins
Q23: The daughter isotope of 203Po when it
Q76: A mixture is not necessarily a solution.
Q84: Decreasing the pressure will _. 2SO₃ (g)
Q105: The order of the reaction with respect
Q108: The typical units for the density of
Q121: Write an expression for the equilibrium constant
Q139: 500 mL of 0.0002 M magnesium sulfate