Predict the effect of increasing pressure in each of the reactions by choosing the appropriate direction shift.
-CH₄ (g) + 2 O₂ (g) 
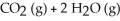
Definitions:
Lessening Competition
refers to actions or agreements that reduce the level of competition in a market, potentially leading to monopolies or oligopolies.
Horizontal Mergers
Mergers between companies that operate in the same industry or market level, often scrutinized for their potential to reduce competition.
Clayton Act
An antitrust law enacted in the United States to promote competition and prevent monopolies by prohibiting certain actions that could lead to anti-competitive practices.
Genetic Traits
Characteristics determined by genes inherited from parents, influencing an individual's physical and possibly behavioral attributes.
Q37: Adding a couple of drops of an
Q45: For the following exothermic reaction:<br>CO + <img
Q58: Adding extra nickel will shift the equilibrium
Q72: Identify the element that was oxidized. 3
Q75: Rate = k[A]3[B]0<br>A)3<br>B)1<br>C)0<br>D)2<br>E)4
Q78: 100 mL of 0.2 M sodium phosphate
Q106: Which of the following acids is a
Q111: "Buffered" solutions are resistant to pH changes,
Q114: Removing sulfur dioxide as it is formed
Q131: 0.16 moles KOH in 2.0 x 10<sup>3</sup>