Based on the following electrochemical cell,what is the standard reduction potential of metal M at 298 K? (R = 8.314 J/K • mol,F = 96500 C/mol) 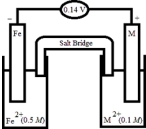 Half-Reaction E° (V) Fe2+(aq) + 2e- → Fe(s) -0.44
Half-Reaction E° (V) Fe2+(aq) + 2e- → Fe(s) -0.44
Definitions:
Depressive Disorders
Mood disorders in which the individual suffers from depression—an unrelenting lack of pleasure in life.
Bipolar Disorder
A mental condition marked by alternating periods of elation and depression, significantly impacting an individual's mood, energy, and daily function.
More Common
Refers to occurring frequently or being prevalent in a particular context or environment.
Anorexia Nervosa
An eating disorder characterized by an obsession with body weight or shape, leading to severe restrictions on food intake and often significant weight loss.
Q9: What is the layer of the atmosphere
Q10: Which electrochemical cell pictured below corresponds to
Q30: What are the secondary pollutants from automobile
Q37: The numbers of geometrical isomers and optical
Q43: An increase in the amount of particulate
Q79: Petroleum is a fossil fuel containing many
Q114: For the endothermic reaction A<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6485/.jpg"
Q122: The radiochemist,Will I.Glow,studied thorium-232 and found that
Q124: For the reaction HCONH<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6485/.jpg" alt="For
Q125: What is the pOH of a 0.025