For the endothermic reaction A2(g)  2A(g) ,a snapshot of an equilibrium mixture of A(g) and A2(g) may look as follows.(Each circle represents 1.0 mol of A atoms,and the volume of the box is 1.0 L.)
2A(g) ,a snapshot of an equilibrium mixture of A(g) and A2(g) may look as follows.(Each circle represents 1.0 mol of A atoms,and the volume of the box is 1.0 L.) 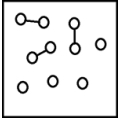 What is the equilibrium constant Kc for this reaction at 298 K?
What is the equilibrium constant Kc for this reaction at 298 K?
(R = 0.08206 L • atm/K • mol)
Definitions:
Interim Financial Statements
Financial reports that cover a period of less than a full fiscal year, providing a view of the company's financial position and operations during that shorter period.
Operating Segments
Parts of an enterprise where separate financial reports are available, regularly examined by the top executive responsible for making operational decisions, to aid in the allocation of resources and the evaluation of performance.
Q11: mL of 3.0 M NH<sub>3</sub>?<br>(Kf for Ag(NH<sub>3</sub>)<sub>2</sub><sup>+</sup>
Q28: Below is a representation of an aqueous
Q62: What type of polymers' shape is determined
Q66: A 0.271-g sample of an unknown vapor
Q68: Which is the half-reaction at the cathode
Q72: Solid ammonium hydrogen sulfide is introduced into
Q84: Which is a Lewis acid?<br>A)CH<sub>3</sub>NH<sub>2</sub><br>B)BCl<sub>3</sub><br>C)F<sup>-</sup><br>D)BF<sub>4</sub><sup>-</sup><br>E)CH<sub>4</sub>
Q99: Which of the following species can act
Q107: Suppose 25.0 g of HI(g)is injected into
Q116: The solubility of the oxidizing agent potassium