The following is an Arrhenius plot of a first-order reaction.The rate constant is measured in units of s-1. 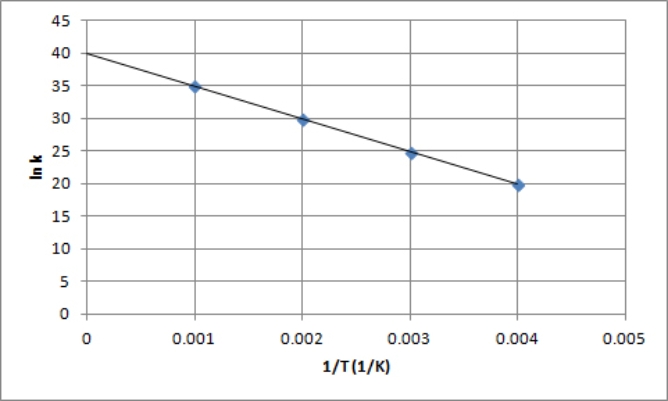 Based on this Arrhenius plot,what is the activation energy of the reaction? (R = 8.314 J/K •mol)
Based on this Arrhenius plot,what is the activation energy of the reaction? (R = 8.314 J/K •mol)
Definitions:
Factoring
A financial transaction and a type of debtor finance where a business sells its accounts receivable to a third party (the factor) at a discount.
Net Working Capital
measures a company's short-term financial health by subtracting its current liabilities from its current assets, indicating the liquidity available to fund day-to-day operations.
Accounts Receivable
Unsettled financial obligations to a corporation from its customers for products or services already delivered or engaged in, awaiting payment.
Accounts Payable
Money owed by a business to its suppliers shown as a liability on the company’s balance sheet.
Q4: The following diagram represents the first-order decomposition
Q8: Ammonia reacts with oxygen to form nitrogen
Q9: The _ is the amount of product
Q19: Which of the following is not true
Q29: Which of the following should be avoided
Q30: Which of the following is an example
Q89: Sucrose decomposes to fructose and glucose in
Q96: A(n)_ _ is the name given for
Q100: Explain the difference between an extensive property
Q107: Which of the following is a chemical