The following is an Arrhenius plot of a first-order reaction.The rate constant is measured in units of s-1. 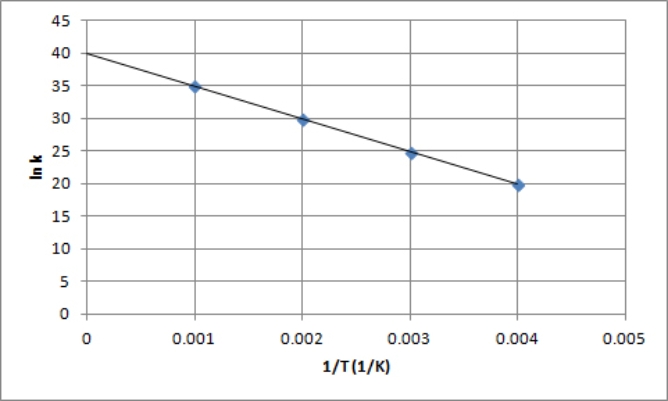 Based on this Arrhenius plot,what is the activation energy of the reaction? (R = 8.314 J/K •mol)
Based on this Arrhenius plot,what is the activation energy of the reaction? (R = 8.314 J/K •mol)
Definitions:
Business Judgment Rule
A legal principle that protects corporate directors or officers from liability for decisions made in good faith and believed to be in the best interest of the company.
Property Taxes
Taxes assessed on real estate by local government, calculated based on the assessed value of the property.
Corporation Liability
Legal concept where a corporation, a legal entity recognized by law, can be held legally responsible for acts or omissions.
Revised Model Business Corporation Act
A set of model statutory provisions designed to guide states in the development of their corporate statutes, providing a standard for corporate governance and operations.
Q9: Which of the following statements is true
Q11: It is recommended to switch from serving
Q15: Process-oriented activities:<br>A)allow children to participate without the
Q22: Biological hazards include all of the following,except:<br>A)Allergens<br>B)Botulism<br>C)Escherichia
Q25: A teacher should do which of the
Q26: Special efforts for disease prevention include all
Q28: Children with which of the following disorders
Q72: The activation energy for the following first-order
Q78: How many grams of Cl<sub>2</sub> can be
Q128: Which of these quantities represents the largest